This is the 3rd report about the decontamination by nano silver which may be LENR transmutation.
In Japan, city garbage is gathered by trucks and taken to the incinerator plant everyday. In the plant, the garbage is burned and the ashes are buried into landfills. That was before March 11, 2011, the disaster of Fukushima Daiichi Nuclear Plant.

After the disaster, at some incinerator plants, the radioactivity of the ashes is very high and exceeds the limit for land reclamation. As the volume of the ashes is about 1-10% of the original garbage, the radioactive materials are concentrated. The highly-radioactive ashes are packed into containers and moved to a temporary warehouse.
For example, in the second incinerator plant of Kashiwa city in Japan, 57.60 tons of radioactive ashes were moved to a temporary warehouse in April of 2013, as below (this data is published at here ).
|
date |
quantity of |
number of |
density of radioactive materials (Bq/Kg) |
|
April 8 |
7.20 |
12 |
38,100 |
|
April 10 |
7.20 |
12 |
49,900 |
|
April 12 |
7.20 |
12 |
49,900 |
|
April 15 |
7.20 |
12 |
47,800 |
|
April 17 |
7.20 |
12 |
51,200 |
|
April 19 |
7.20 |
12 |
51,200 |
|
April 24 |
7.20 |
12 |
49,400 |
|
April 26 |
7.20 |
12 |
49,400 |
|
sum |
57.60 |
96 |
51,200 (Max) |
On March 28, 2012 from 9:30 am to 11:45 am, Dr. Norio Abe, Chief of Itabashi Firefly Ecosystem Center, went to the plant and conducted an experiment that would decontaminate the ashes using nano-silver.
A report on the experiment is shown in SlideShare ( here ). I added English terms beside the Japanese terms. Figures in the report are not as clear in the embedded view of SlideShare, so please download the PDF document from SlideShare if you have trouble reading it.
Abstract of the experiment:
- 12Kg of the ashes were moved to 3 pails ( Each pail had 12Kg of the ashes)
- The team prepared 3 types of materials. They mixed each material for each pail and measured radioactive level just after mixing and after 30 min ( or 25 min ).
- The 3 types of materials:
(A) 3.6 liter of tap water ( for controlled experiment )
(B) 3 liter of nano silver embedded collagen (10ppm) and 3 Kg of nano silver embedded boan coal
(C) 3 liter of nano silver embedded collagen (20ppm) and 3 Kg of nano silver embedded boan coal
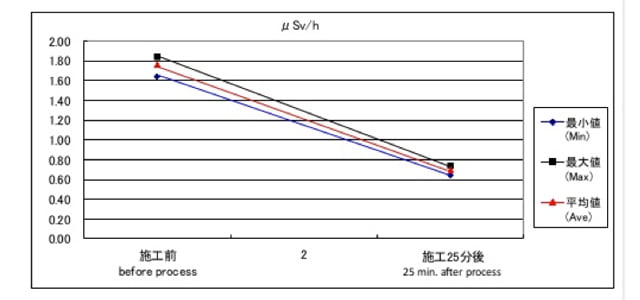
(The difference between B and C is the density of nano silver embedded collagen — 10 ppm vs 20 ppm) - As the result, in the cases of B and C, the level of radioactivity of the ashed decreased. For example, in the case of C, the level was 6.12 μSV before mixing and decreased to 4.00 μSV just after mixing. The level after 30 min was similar to the level just after mixing.
Unfortunately, they had to end the experiment after 30 min of mixing due to time limitations. I speculate that the level of radioactivity might be decreased further by additional stirring of ashes, because in the experiment (here) reported at the conference of “radiation detectors and their uses”, a decrease after stirring is shown.
Anyway, I think this experiment shows the possibility of transmutation from radioactive material into a non-radioactive one. I hope other researchers will try and re-produce this experiment.
If you want to contact me, please write a comment or send e-mail to me ( sengaku1046@gmail.com ).
Cold Fusion Now!
Related article:
- Nanoscale Ag may decrease radiation of Cesium 134 and 137 by LENR transmutation?
- Field work of Cesium decontamination by nano silver

It’s an aqueous solution so there are protons from water (a trace of deuterium).
Can’t think of anything that isn’t crazy (as usual) but the following reactions would be exothermic.
H(1) + Cs(134) > He(4) + Xe(131) 6.39 MeV
H(1) + Cs(137) > He(4) + Xe(134) 6.44 MeV
I wonder if anything happens with heavy water (D2O).
H(2) + Cs(134) > He(4) + Xe(132) 13.1 MeV
H(2) + Cs(137) > He(4) + Xe(135) 10.6 MeV
Could something similar to the Iwamura transmutation experiments be taking place but instead of deuterium gas it’s light water and instead of palladium it’s on silver?
http://indico.cern.ch/getFile.py/access?resId=5&materialId=slides&confId=177379
Dear Alan,
Thank you for your suggestion.
As Dr. Shin Iwasaki and Dr. Norio Abe did not measure the elements included by the ashes yet, I am sorry that I can not examine your hypothesis. I will tell it to them.
Hi Toshiro: Thank you for bring these fascinating observations of Dr. Shin Iwasaki and Dr. Norio Abe to our attention.
I was just trying to think of reactions that would not have any radioactive products. Even if my crazy idea is correct, the products are helium and xenon that are normally found in air anyway and therefore almost impossible to detect. This is just so interesting that I had to give it some thought. Hopefully some smart people might come up with a better answer. Has this been brought to Chris Busby’s attention? I’m sure he’d be fascinated by this. This is Dr. Busby’s video (WARNING: some people might find this disturbing.) http://www.youtube.com/watch?v=4iutbbfduAQ
So if this was LENR transmutation, it would presumably make some heat-energy too, right? It does not appear to have temperature data included, I suppose they are just measuring radioactivity. But it is actually disappearing, right? Like not being transferred somewhere else, but going down.
Here’s those Mitsubishi slides:
Ruby san,
Thank you for your comment and presentation made by Dr. Iwamura.
I agree that it would make some heat energy if it was LENR transmutation.
But, in this case, the volume of heat may be very small because the volume of radioactive Cesium is very small.
I hear that the weight of Cesium 137 included in 10,000 Bq material is equal to 3.12274E-09 gram. Even if LENR makes greater heat than chemical reaction, it must be not easy to measure the heat generated by 3.12274E-09 gram of Cesium 137. We have to invent another measurement method…
Yes, I see, it would be tough to measure. Well, at least it’s effecting radioactivity – whatever is happening.
Hi Ruby: Good question. I like Toshiro quantitative answer. So, it doesn’t rule out a LENR.
Thank you very much
It is unclear to me if the silver is transmuted.
I did notice the production of Platinum from tungsten with interest.
Does an explanation exist for this observation, or was this an experiment?
Alan, You did not mention Ag in your equations.
Could the decrease in radioactivity have been an artifact due to shielding? I doubt that the researchers would have overlooked something as basic as that. But given the time constraints how could they have got a uniform sample?
I have checked the link offered and I see that self-screening was taken into account.
Hi Arthur, I just went nuts again. I was just thinking that the reaction might take place on the surface of the silver but that the silver doesn’t react itself because in the case of the Mitsubishi reaction it doesn’t seem that the palladium is being transmuted either but that the palladium is needed for the reaction to take place. It really isn’t a logical thought. It’s just a gut feeling that I have. And yeah, Dr. Shin Iwasaki and Dr. Norio Abe are looking for gamma rays and I think (just from my experience in the dentist chair when they put a lead apron on us for x-rays) that they would be hard to shield.
One last thought. Natural cesium is 100% Cs(133). Maybe run the same experiment with a more concentrated solution of natural cesium (some salt of cesium: Carbonate, chloride, et cetera…) and see if you can detect an exotherm. The following reaction with natural cesium would also be exothermic.
H(1) + Cs(133) > He(4) + Xe(130) 6.67 MeV